Background
Fostamatinib, a spleen tyrosine kinase inhibitor, has been approved for the treatment of chronic immune thrombocytopenia (ITP) in adults in the US, Canada, Europe, Israel, and Japan. We evaluated the long-term efficacy and safety of fostamatinib treatment and the off-therapy platelet count in Japanese patients with primary ITP in a phase 3 clinical trial with a 24-week double-blind period, a 28-week open-label extension period, and a pre-defined washout period.
Methods
The trial enrolled Japanese patients who had failed to respond or did not tolerate ≥1 prior ITP treatment, who had a mean platelet count <30,000/μL (based on 3 screening and baseline platelet measurements), and had a platelet count at each visit <35,000/μL. Dosing was at 100 mg bid for 4 weeks and then 150 mg bid if needed and tolerated. One concomitant treatment was allowed (corticosteroids, azathioprine or danazol). The dosage of the concomitant treatment was to remain the same during the 24-week double-blind period but could be reduced or discontinued during the open-label period.
Efficacy endpoints were platelet response rate (i.e., the percentage of patients who achieved a platelet count of ≥ 50 × 10 3/μL at 2 consecutive visits at least 28 days apart). The platelet count of the responders during the study period were evaluated.
Results
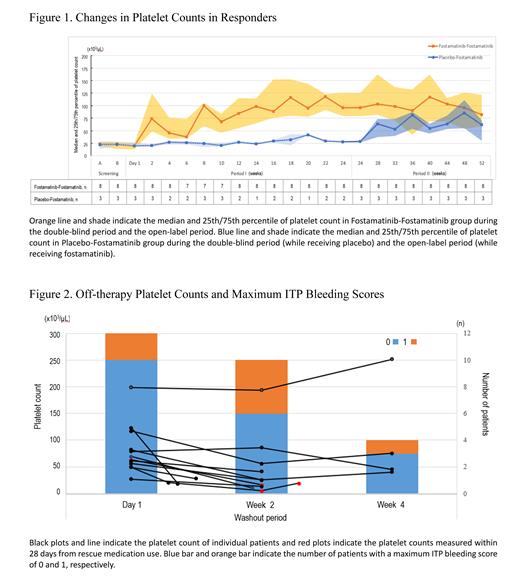
A total of 33 patients who were either treated with fostamatinib in the 24-week double-blind period (Fostamatinib-Fostamatinib group, n=22) or treated with placebo in the double-blind period and then treated with fostamatinib in the 28-week open-label extension period (Placebo-Fostamatinib group, n=11) were analyzed. Of those, 79% (26/33) were women, the median age was 62, the median baseline platelet count was 19 x 10 3/μL, and 58% (19/33) had two or more previous treatments. The platelet response rate was 36% (8/22) in the Fostamatinib-Fostamatinib group and 27% (3/11) in the Placebo-Fostamatinib group. The platelet count of the responders in the Fostamatinib-Fostamatinib group increased to ≥50 × 10 3/μL shortly after initiating fostamatinib and remained around 100 × 10 3/μL between Week 14 and Week 52, whereas the platelet count of the responders in the Placebo-Fostamatinib group remained low while receiving placebo but increased after receiving fostamatinib ( Figure 1). Among 14 patients with concomitant glucocorticoids treatments at the beginning of the open-label period, 43% (6/14) reduced or discontinued glucocorticoids while receiving fostamatinib without disease relapse.
In the washout period of up to 4 weeks, all 12 patients experienced a mild decrease in platelet count, but none developed bleeding events ( Figure 2). Three of four patients who completed the 4-week washout period had a slight increase in platelet counts from Week 2 to Week 4.
In the double-blind and open-label periods, adverse events were reported in 96% (21/22) of the Fostamatinib-Fostamatinib group and 100% (11/11) of the Placebo-Fostamatinib group, and treatment-related adverse events in 77% (17/22) and 46% (5/11), respectively. In the washout period, adverse events were reported in 50% (6/12) of patients, and treatment-related adverse events in 0% (0/12). We found no new safety risk or late-onset adverse events specific to Japanese patients.
Conclusions
The long-term efficacy and safety of fostamatinib were observed in Japanese patients with primary ITP, along with the feasibility of glucocorticoid reduction/discontinuation during fostamatinib treatment, and a lack of bleeding events after abrupt discontinuation of fostamatinib. The findings obtained from this study will help position fostamatinib as a second-line treatment in patients with primary ITP.
Disclosures
Ito:Bristol-Myers Squibb Company: Honoraria, Research Funding; Asahi Kasei Pharma Corporation: Research Funding; Chugai Pharmaceutical Co., Ltd.: Honoraria, Research Funding; Kyowa Kirin: Research Funding; Mundipharma: Honoraria; Novartis: Honoraria; Takeda Pharmaceutical Company Limited: Honoraria; AbbVie GK.: Honoraria; Nippon Shinyaku: Honoraria; Eisai: Honoraria; CSL Behring: Honoraria; Sanofi: Honoraria. Hatta:Kyowa Kirin: Honoraria; Chugai: Honoraria; Janssen Pharma: Honoraria; Bristol‐Myers Squibb: Honoraria; Takeda: Honoraria; Ono Pharma: Honoraria. Fujimaki:AbbVie GK: Honoraria; Novartis: Honoraria; Pfizer: Honoraria; Bristol-Myers Squibb: Honoraria; Otsuka Pharmaceuticals: Honoraria; Janssen Pharmaceutical KK: Honoraria; Nippon Shinyaku: Honoraria; Chugai Pharmaceutical Co., Ltd.: Honoraria; Meiji Seika Pharma Co., Ltd.: Honoraria; Takeda Pharmaceutical Company Limited: Honoraria; CSL Behring K.K.: Honoraria. Shichiri:Kissei Pharmaceutical Co., Ltd.: Current Employment. Saotome:Kissei Pharmaceutical Co., Ltd.: Current Employment. Masuda:Rigel Pharmaceuticals, Inc.: Current Employment, Current equity holder in publicly-traded company. Tomiyama:Novartis: Honoraria; Sysmex: Consultancy; Kyowa Kirin: Honoraria; Kissei Phamaceutical: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal